 | | | | |  | By Megan R. Wilson | Did someone forward? Sign up here to get this newsletter in your inbox. In today’s issue: What you can find on TrumpRx. … GoodRx partners with the government’s direct-to-consumer platform, providing patients access to thousands of pharmacies. … A diagnostic test touted in an upcoming Super Bowl ad has questionable reliability. TGIF. And thanks for checking in with Health Brief. → BREAKING as we were about to press send on the newsletter: The top lawyer at the Department of Health and Human Services said it has referred Hims & Hers Health to the Justice Department, citing “potential violations” of the “Federal Food, Drug, and Cosmetic Act and applicable Title 18 provisions." Title 18 is the federal criminal code, underscoring the potential severity of the referral. This comes following the company’s announcement on Thursday of a compounded Wegovy pill knockoff. On Thursday afternoon, the head of the Food and Drug Administration sent a warning on social media to “companies mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products. Hims & Hers was not immediately available for comment. Do you have any story tips or health policy intel? Are you FDA Commissioner Marty Makary? Shoot me a note at megan.wilson@washpost.com. If you prefer to message me securely, I’m also on Signal at megan.434. This newsletter is published by WP Intelligence, The Washington Post’s subscription service for professionals that provides business, policy and thought leaders with actionable insights. WP Intelligence operates independently from the Washington Post newsroom. Learn more about WP Intelligence. | 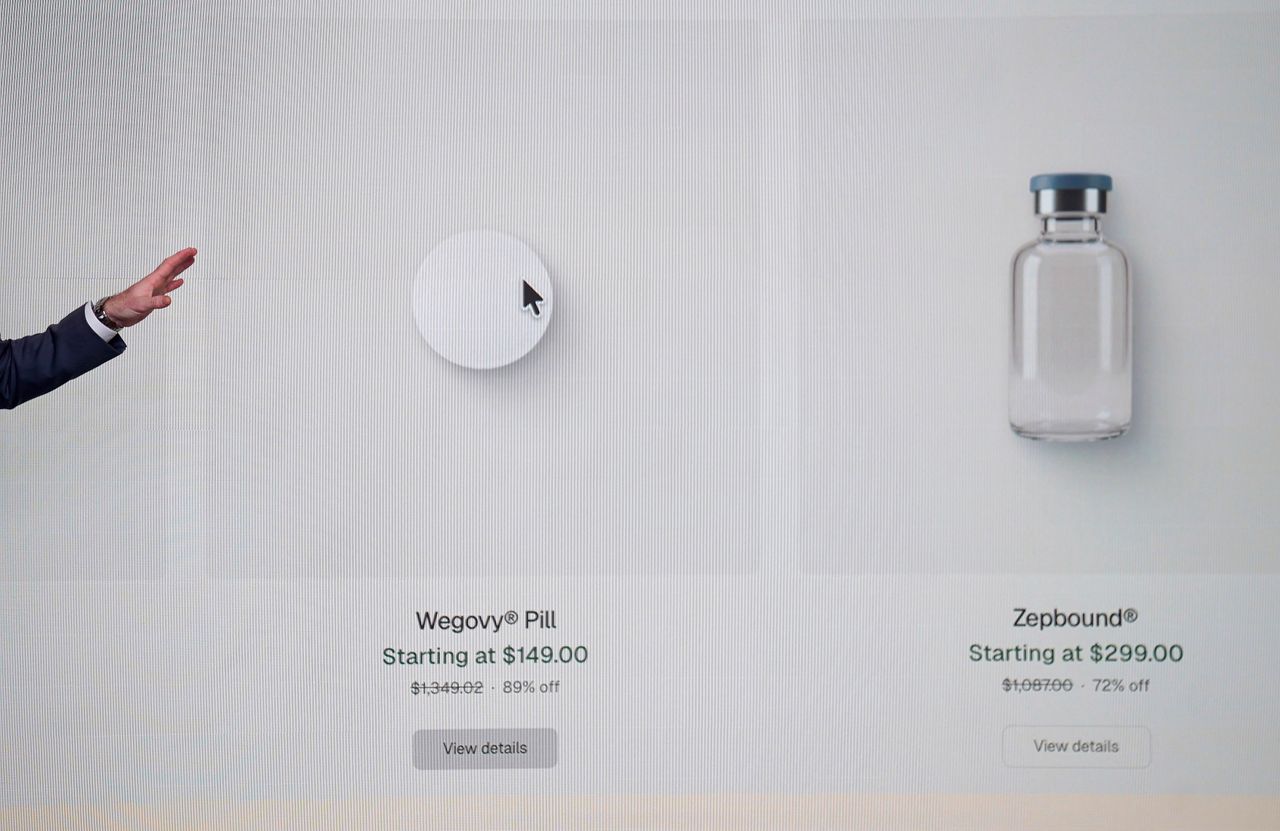 | | Joe Gebbia, the chief design officer of the National Design Studio, points to a display during the launch of TrumpRx. (Nathan Howard/Getty Images) | | | |  | The Lead Brief | After months of buildup and deals with drugmakers, President Donald Trump unveiled TrumpRx Thursday evening. But for many of the medications listed, Americans can already find the same drugs for far less money outside the platform. TrumpRx doesn’t sell medications directly. It’s a slick website that guides consumers to platforms hosted by pharmaceutical companies — and where people are able to purchase drugs at a discount or, in some cases, apply for patient assistance programs. For some of the medications listed on TrumpRx, consumers are able to take a GoodRx coupon to the pharmacy of their choice for a prescribed medication listed on the platform. Why it matters: Trump has attempted to implement so-called most-favored nation (MFN) pricing policies — which involve drugmakers lowering prices in the U.S. to align with other wealthy nations — since his first term. Trump sent letters to 17 large drugmakers last year, along with a list of demands including directly offering Americans discounted drugs if they want to pay cash. Although the White House has made the drug-pricing deals a large part of affordability messaging in the run-up to critical midterm elections, experts question how much of an impact this could have for most Americans. Most of the medications are likely cheaper through their insurance or have cheaper generic versions available. Sixteen companies — including Merck, Bristol Myers Squibb and Novartis — have cut MFN drug pricing deals with the Trump administration, and thus TrumpRx.gov was born. At the time of the platform’s launch on Thursday, just five drugmakers had their products listed on TrumpRx: Pfizer, AstraZeneca, EMD Serono, Novo Nordisk and Eli Lilly. “The fact that the first five companies we struck deals with are right now the only five companies whose products are on the site is not a coincidence,” said an administration official, who was granted anonymity to share additional details about the platform. More medications will be added to TrumpRx “in short order and continually as we hash that out with the drugmakers whose products have not been listed yet,” the official tells me. The process “naturally takes time.” Here are three key numbers behind the initial drugs on TrumpRx: 43: This is how many medications are currently listed on the platform. 31: This is how many of the medications listed on TrumpRx are manufactured by Pfizer. 20: The number of Pfizer’s offerings on TrumpRx that have generic competition, almost all of which can be found at a fraction of the drugmakers’ discounted cost. SOME EXAMPLES: - Protonix, a drug that treats acid-related conditions such as acid reflux, is listed on TrumpRx at $200 for a 30-day supply. A generic version is available on Mark Cuban’s Cost Plus Drugs platform for about $6 (plus $5.25 for shipping).
- Chantix, the smoking-cessation medication, is $106 on TrumpRx for a 60-day supply. But there’s also a generic version available at Cost Plus Drugs for about $10 for the same quantity.
- Tikosyn, a medication that helps people with atrial fibrillation (AFib), is listed as $336 for 60 capsules on TrumpRx. Generic versions are available elsewhere for as little as $12 for the same number of pills.
“TrumpRx offers branded products. For those branded products, TrumpRx offers the lowest prices,” the Trump administration official told me in response to questions about why other, cheaper options aren’t listed. “It’s entirely possible that there are generic products available that are cheaper than the branded products available on TrumpRx, but many products already on the site do not have generic competition.” The official pointed to expensive drugs — such as GLP-1 weight loss drugs and fertility drug Gonal-F — which are offered on the platform and don't have generic competition. → Deals that drugmakers cut with the administration are strictly confidential, so it’s not fully known which of each company’s products will be covered by the pricing arrangements. Congressional Democrats have blasted the platform, including Sen. Ron Wyden (Oregon), the top Democrat on the Senate Finance Committee. He called it “nothing more than a glorified coupon book.” | | | |  | Industry Rx | A key part of TrumpRx is its connection with GoodRx, a company that offers coupons for medications that patients can use instead of insurance. The company calls itself “a core integration partner” for the administration’s new platform. Patients can find the company’s coupons for many medications on the site, allowing them to fill prescriptions wherever they choose. GoodRx said in a release that it has a network of 70,000 pharmacies nationwide. → As Health Brief has reported, this had been a key concern for retail pharmacies, which worried that patients could fill medications from online pharmacies after securing their prescription from drugmakers’ platforms. When asked whether it receives payments from drugmakers, the federal government — or both— in connection with its inclusion on TrumpRx, a spokesperson for GoodRx tells me that “financial relationships are strictly with our pharmaceutical partners.” “GoodRx enables pharma manufacturers to rapidly deploy and scale emerging pricing models with minimal operational lift,” the company said in a release. | | | |  | Lobbying Ledger | Last year, GoodRx revived their lobbying operation, which had been dormant since the middle of 2023 when the company dropped all its lobbyists. In April, it brought on Gary Kline to lead its Washington office, hired multiple outside lobbying firms, and shelled out almost $1 million on lobbying by the end of 2025. One of those firms — Capitol Hill Consulting Group — disclosed lobbying the Centers for Medicare and Medicaid Services, an agency actively engaged in the MFN drug price negotiations with drugmakers, throughout the year. During the last three months of 2025, disclosures say lobbyists at Capitol Hill Consulting Group discussed “issues related to direct to consumer access” and “ways to lower prescription drug costs.” Another firm, Continental Strategy, disclosed lobbying the Executive Office of the President on digital “platforms and healthcare costs” toward the end of last year. Neither firm responded to a request for comment. GoodRx said that it doesn’t “discuss specific lobbying strategies.” “Companies have lobbied the federal government for various reasons for decades,” the administration official responded in an email when asked about the contacts. “Unless you can pinpoint an exact way the Administration sought to give an undue handout or business opportunity for GoodRx — which you cannot — then I don’t see the point of including this in your writeup.” “The only thing we care about is drugmakers offering drugs at MFN prices to patients via TrumpRx,” said the official. | | | |  | Market Moves | — Adapted from a longer article written by Daniel Gilbert for The Washington Post. Part of the commercial that Hims & Hers Health plans to air during the Super Bowl features a cutting-edge blood test designed to detect more than 50 types of cancer before symptoms show up. The ad features a man pulling out his phone as he waits in a car, checking a screen that reads “No cancer signal detected.” He smiles and closes his eyes in an expression of relief. However, the test’s reliability is questionable, and even Grail — the company that makes it — advises patients to follow up with additional medical procedures to diagnose cancer. Why it matters: The Super Bowl ad is part of Hims & Hers’ larger ambition to turn the conventional, doctor-driven, insurance-based medical system on its head by giving consumers a menu of on-demand tests and treatments to improve their health. But the test’s portrayal in the advertisement is raising concerns among some experts, who say it presents the risk of giving patients a false sense of security. - A large clinical trial showed that the test, called Galleri and based on a single blood draw, is not always reliable — especially on its own. It missed more cases of cancer than it discovered, according to Eric Topol, director of Scripps Research Translational Institute, who analyzed the trial results on his Substack in October.
“A lot of people, they might not be so savvy about interpreting the test,” Topol told Daniel in an interview. “They get the result ‘negative, no cancer detected.’ Why would they chase that down further?” - Grail has said that adding it to standard screening for breast, cervical, colorectal and lung cancers led to a sevenfold increase in cancers detected. Grail says Galleri is recommended for adults “with an elevated risk of cancer,” which it considers to include people who are at least 50 years old.
Patrick Carroll, chief medical officer for Hims & Hers, told Daniel that the telehealth company will tell customers whom the test is made for, but won’t limit who can get a prescription. Some cancers, such as prostate and colorectal cancer, can strike earlier than age 50, Carroll pointed out. “We’re really empowering the patient more to take control of their own health,” he said. | | | |  | Litigation Report | In a victory for hospital groups, the Trump administration is scuttling an initiative targeting the 340B discount drug program that allows providers to receive cheaper medications from drugmakers. The pilot program — which drew a legal challenge from the hospital industry — would have required providers in the program to pay the full price for medicines before receiving a rebate later. → In a legal filing on Thursday, the Department of Health and Human Services said that further litigation would not be “fruitful.” “The AHA appreciates HHS’ decision to go back to the drawing board and rethink its Rebate Program,” said Rick Pollack, who leads the American Hospital Association, in a statement. The action comes just weeks after a federal appeals court upheld a freeze on the pilot program. - The pilot program was an effort to ensure that drugmakers aren’t giving discounted drugs to facilities that don’t qualify for them. However, providers say that requiring full payment in advance could cause some cash-strapped hospitals or clinics to close. It was supposed to begin on Jan. 1.
- The 340B program was designed to enable safety-net providers that treat a large number of low-income or uninsured patients to stretch limited resources by compelling drugmakers to provide steep discounts on medication. Critics argue that it’s being abused by some facilities to bolster profits or subsidize other operations without benefitting patients.
What’s next: Should HHS decide to take a different approach to tinkering with the 340B program, the agency said that it would first solicit public comment on any proposal. Tom Kraus, vice president for government relations at the American Society of Health-System Pharmacists, also hailed the decision. “Providers should not shoulder increased costs for programs intended to require manufacturer discounts,” Kraus said, arguing that the scuttled pilot was “unworkable” and a “threat” to the 340B program’s integrity. | | | | |