|
 |
Welcome to Popular Information, a newsletter dedicated to accountability journalism.
Earlier this year, Popular Information broke the news that Extremity Care – a company that sells bandages to Medicare for thousands of dollars per square inch – donated $5 million to MAGA Inc., President Trump’s super PAC, on February 24. Six days later, Trump blasted a pending Biden administration rule that would have barred Medicare from covering Extremity Care’s products.
The Biden rule was initially scheduled to take effect in February 2025, but was delayed by the Trump administration until April 13 as part of a blanket regulatory freeze. On April 11, 40 days after Trump’s post, the Centers for Medicare & Medicaid Services (CMS) announced the rule would be delayed until at least January 1, 2026, allowing Extremity Care to continue charging Medicare for its products.
As that deadline approaches, Extremity Care has secretly donated $2.5 million toward the construction of a new White House ballroom, a top priority for Trump. The White House publicly released a list of donors last month, but Extremity Care was not included. According to The New York Times, the company was permitted to donate anonymously.
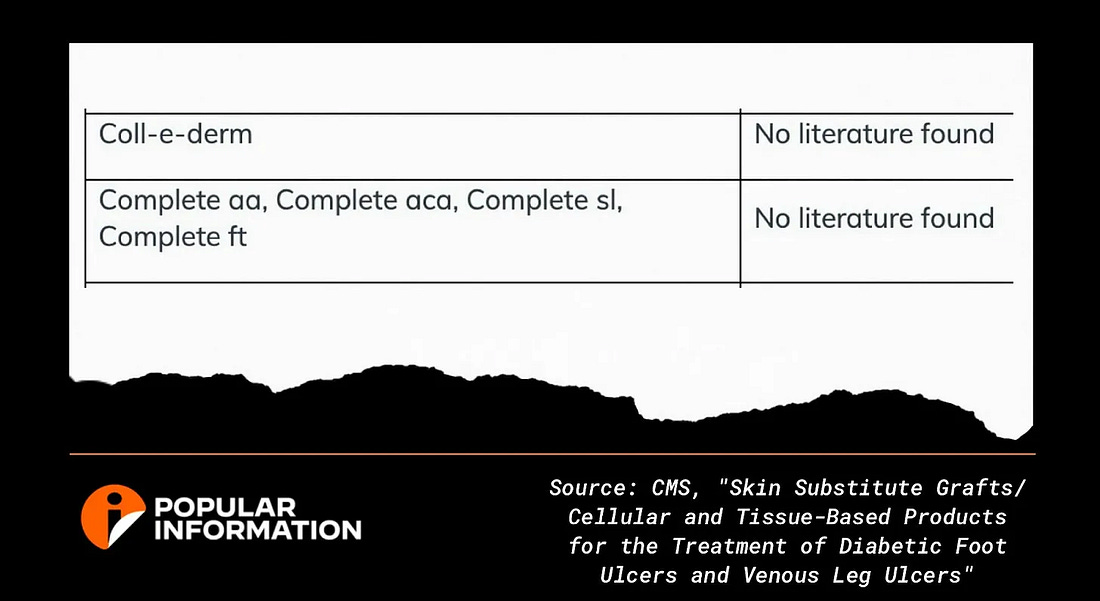
CMS, in preparation for implementing the Biden rule, determined that 17 skin substitute products have proven their effectiveness in scientifically valid studies. The agency found that Extremity Care products lack scientific backing. One of Extremity Care’s products, Coll-e-Derm, costs $11,051.10 per square inch. Another Extremity Care product, CompleteFT, costs $7,812.38 per square inch.
Skin substitute products went from a negligible source of Medicare spending in 2014 to $256 million in 2019, and then surged to more than $10 billion in 2024. As a result of the Trump administration’s delay of the rule, Medicare spending on skin substitutes “is projected to reach $15.4 billion by the end of 2025.”
“The deliberate misuse of skin substitutes is endangering America’s seniors and pricing schemes are undermining the integrity and the long-term sustainability of Medicare,” said Emily Brower, CEO of the National Association of Accountable Care Organizations. “If we are truly committed to combating fraud, waste, and abuse, we must put an end to this exploitation by bad actors.”
Many of the products with scientific backing are much less expensive than those offered by Extremity Care. Oasis wound matrix, a product that has proved effective in scientific research, charges Medicare $75.51 per square inch. Another scientifically proven product, Apligraf, charges $195.58 per square inch.
On July 14, the Trump administration announced a new proposed Medicare rule for skin substitutes. The new proposal would cap payments for skin substitutes at $806 per square inch. This is lower than what many companies, including Extremity Care and Legacy, currently charge, but it would not limit coverage to products that are scientifically proven to be effective. Further, many of those proven products are available for far less than $806 per square inch.
The Medicare Access to Skin Substitutes Coalition (MASS Coalition), which represents Extremity Care and others in the industry, also opposes the new rule. In a September 12 letter to CMS, the MASS Coalition claims that $806 per square inch “does not cover the many costs incurred by manufacturers to deliver these products to providers who administer them to patients.” They suggest that if the rule goes into effect, manufacturers may stop selling their products, and “access to these critical treatments will be seriously compromised.” Instead, the industry is proposing a payment cap as high as $6,277.41 per square inch, plus additional payments for “innovative” products.
They cite a paper by Dr. William Tettelbach and published in the Journal of Wound Care, which recommends a reimbursement cap of $3,083.86 to $4,541.93 per square inch. The letter does not mention that Tettlebach has been paid by Extremity Care, a subsidiary of Tiger BioSciences, to conduct research.
In addition to writing the letter, the skin substitute industry has had an opportunity to press their case with the Trump administration in person. On October 21, Extremity Care CEO Oliver Burckhardt attended an exclusive dinner with Trump for top donors on the Rose Garden patio.
While some news outlets are turning to AI to reduce reporting time and cut costs, Popular Information is taking the opposite approach. This newsletter is doubling down on in-depth journalism and original research to uncover new information.
That’s why you’ll find news and analysis here that you will not find anywhere else. We do not believe in shortcuts. We believe in publishing stories that matter.
If you value this kind of reporting, you can support it by becoming a paid subscriber.
If you have a news tip, please contact us via Signal at juddlegum.47. We will maintain your anonymity.