|
||
| PRESENTED BY THE HEALTHCARE DISTRIBUTION ALLIANCE | ||
| Axios Vitals | ||
| By Tina Reed, Peter Sullivan and Maya Goldman · Oct 21, 2025 | ||
|
We have lots of news! Today's newsletter is 1,032 words, a 4-minute read. |
||
| 1 big thing: How Lilly became the GLP-1 leader | ||
| By Tina Reed | ||

|
||
|
Illustration: Maura Losch/Axios |
||
|
Eli Lilly is poised to leapfrog Novo Nordisk and become the dominant player in an anti-obesity drug market that could reach $150 billion by the end of the decade. Why it matters: The push to develop blockbuster weight loss drugs had been a two-horse race dominated by Novo Nordisk until the maker of Wegovy and Ozempic started backsliding on weaker sales growth.
State of play: While Novo leads in sales for the moment, Eli Lilly's injectables have shown better efficacy in clinical trials and are said by doctors and patients to be more tolerable, market analysts say.
Yes, but: Last week, Novo got the FDA's nod for its own oral GLP-1 drug, called Rybelsus, to be used for heart disease. It's also awaiting FDA approval of an oral version of Wegovy in early 2026 — the first such oral option for obesity.
What we're watching: How big of an impact oral GLP-1s have in the U.S., whose patients have greater amounts of weight to lose.
|
||
|
|
||
| 2. Exclusive: Dems target "orphan drug" exemption | ||
| By Peter Sullivan | ||

|
||
|
Welch at a June hearing. Photo: Chip Somodevilla/Getty Images |
||
|
Senate Democrats are launching an effort to repeal a portion of Republicans' budget law that exempts certain "orphan drugs" from Medicare price negotiations, citing estimates that the cost of the carve-out has ballooned. Why it matters: The measure targets a provision that the biotech industry argues is important for innovation, but that Democrats say is actually a costly handout to major pharmaceutical companies. Driving the news: The bill, first shared with Axios, would repeal a provision passed in July that expands an existing exclusion for drugs that treat rare diseases from the price talks.
Between the lines: The Democratic bill would set up a new system where rare disease drugs are only exempted from negotiations if they account for less than $400 million in annual Medicare spending.
|
||
|
|
||
| 3. Exclusive: Walmart first to sell OTC glucose monitors in stores | ||
| By Kelly Tyko | ||

|
||
|
Robert Ford, chairman and chief executive officer of Abbott Laboratories, unveils a portfolio of Lingo biosensors at the Consumer Electronics Show in January. Photo: Patrick T. Fallon/AFP via Getty Images |
||
|
Walmart will become the first U.S. retailer to sell an over-the-counter continuous glucose monitor in physical stores, as Abbott's Lingo rolls out to more than 3,500 locations and online, the health care company told Axios exclusively today. Why it matters: It's the latest sign that health tech is going mainstream — making tools that previously required a prescription more accessible to consumers. Driving the news: Abbott said Walmart is the first brick-and-mortar retailer to carry its Lingo device, previously available only at HelloLingo.com and Amazon.
Zoom in: Abbott calls Lingo its first consumer biowearable, making continuous glucose tracking — once limited to people with diabetes — available to anyone interested in monitoring their levels without needing to do multiple finger pricks.
|
||
|
|
||
|
A MESSAGE FROM THE HEALTHCARE DISTRIBUTION ALLIANCE |
||
| Creating American jobs, powering healthcare | ||
|
|
||
|
Healthcare distributors support hundreds of thousands of American jobs, fueling local economies. Their impact: Distributors employ skilled workers who are responsible for picking, packing and shipping lifesaving medicines. |
||
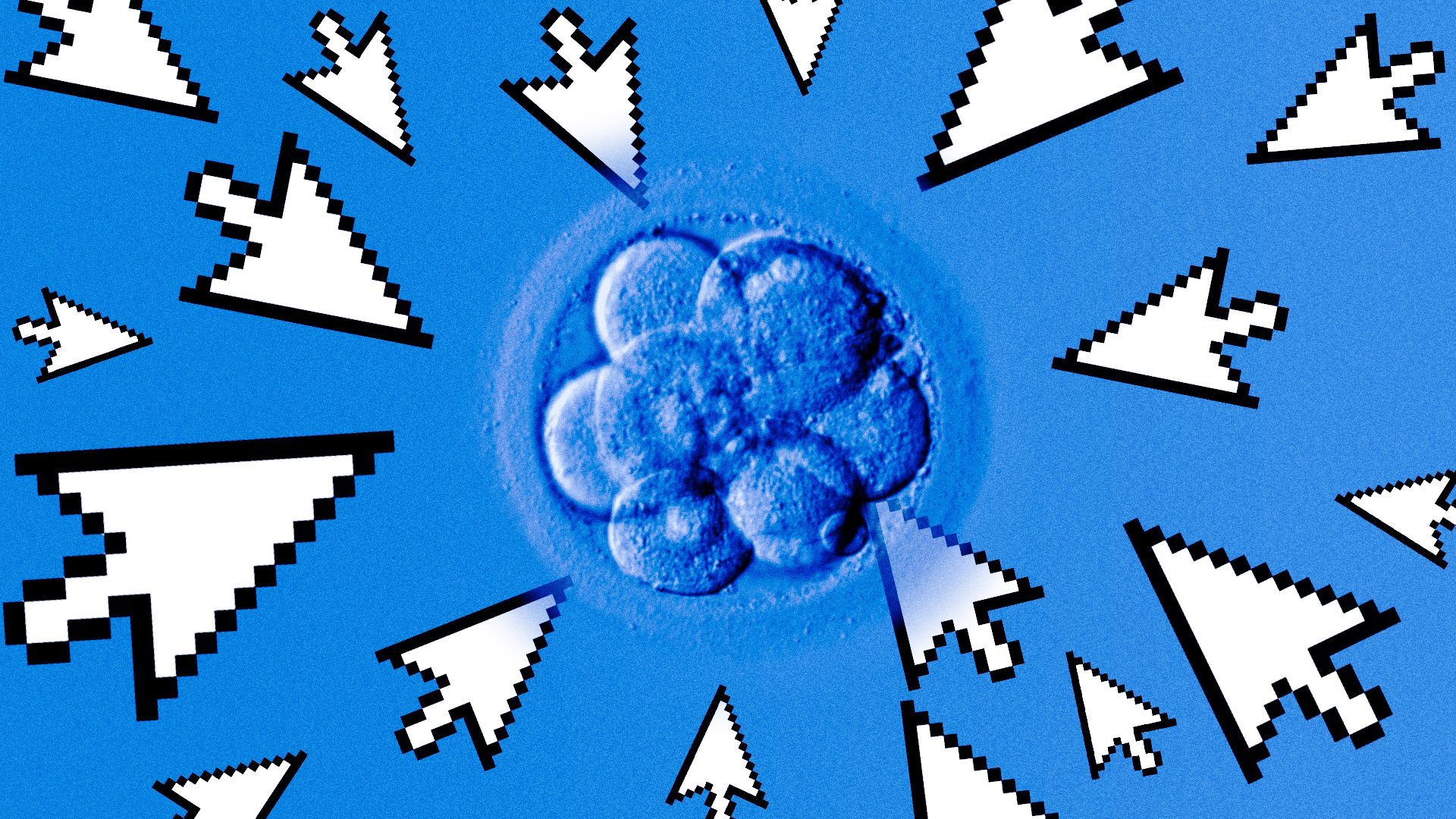
| 4. Exclusive: Startup will use AI to assess embryo disease risk | ||
| By Ashley May | ||
|
||
|
Illustration: Aïda Amer/Axios |
||
|
A genetic testing startup launched an AI genomics research arm today aimed at predicting the likelihood that IVF embryos will develop certain cancers, Alzheimer's and other chronic diseases. Why it matters: Nucleus Genomics is taking preventative health to an entirely new level: before birth.
Driving the news: The company will use DNA analysis to scan for Alzheimer's, breast cancer, coronary heart disease, endometriosis, hypertension, prostate cancer, arthritis, and Type 1 and Type 2 diabetes.
Nucleus Labs is a medical lab that is subject to the same regulations as other clinical genetic testing.
|
||
|
|
||
| 5. Catch up quick | ||

